Want to use a PH tester? Read this guide first
Unlocking the Secrets of pH Measurement: Exploring the Pros and Cons of pH Meters
In the vast realm of scientific analysis and industrial applications, pH measurement stands as a fundamental pillar of understanding acidity and alkalinity. At the heart of this crucial analysis lies the pH meter, a remarkable instrument that has revolutionized the way we assess and monitor pH levels in various solutions. With its ability to provide accurate and instantaneous readings, pH meters have become indispensable tools in laboratories, research facilities, manufacturing processes, and environmental monitoring.
However, like any scientific instrument, pH meters have their strengths and weaknesses. In this exploration, we delve into the pros and cons of using pH meters, shedding light on the advantages that make them invaluable assets, as well as the challenges that users may encounter along the way.
Join us on this journey of discovery as we unlock the secrets of pH measurement, uncovering the benefits that enable precise analysis and decision-making, while also acknowledging the limitations that demand careful attention and maintenance. Whether you are a seasoned scientist, a quality control professional, or an inquisitive learner, this exploration aims to equip you with a comprehensive understanding of the potential and pitfalls of pH meters.
Prepare to dive into the world of pH measurement, where accuracy, versatility, and speed intertwine with cost considerations, maintenance requirements, and the delicate nature of pH electrodes. By exploring the pros and cons, you will gain a deeper appreciation for the power and complexities of pH meters, ultimately empowering you to make informed choices and maximize the potential of this indispensable tool.
So, let us embark on this captivating journey to unlock the secrets of pH measurement and uncover the hidden facets of pH meters. Together, we will unravel the mysteries, weigh the pros and cons, and navigate the realm of pH analysis with confidence and precision.
What does the "pH" mean?
The term "pH" stands for "potential of hydrogen." It is a measure of the acidity or alkalinity of a solution. The pH scale ranges from 0 to 14, with 7 being considered neutral. Solutions with a pH below 7 are considered acidic, while solutions with a pH above 7 are considered alkaline (basic).
The pH scale is logarithmic, which means that each whole number change on the scale represents a tenfold difference in acidity or alkalinity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5.
The pH of a solution is determined by the concentration of hydrogen ions (H+) present in the solution. Acidic solutions have a higher concentration of hydrogen ions, while alkaline solutions have a lower concentration of hydrogen ions and a higher concentration of hydroxide ions (OH-).
pH is an important parameter in various scientific disciplines, including chemistry, biology, environmental science, and medicine. It is commonly measured using pH meters or pH indicator papers to determine the acidity or alkalinity of a solution.

Fig 1. Protable Digital PH Meter Tester Aquarium Pool Water Wine Urine LCD Pen Monitor
How can measure the pH?
The pH of a solution can be measured using various methods. Here are three common techniques for measuring pH:
- pH Meter: A pH meter is a device specifically designed to measure the pH of a solution accurately. It consists of a pH probe, which is an electrode that generates a voltage proportional to the hydrogen ion concentration in the solution. The pH probe is connected to a pH meter, which displays the pH value based on the voltage reading. pH meters are widely used in laboratories and scientific research settings.
To measure pH using a pH meter, you typically follow these steps:
a. Calibrate the pH meter using standard buffer solutions with known pH values.
b. Immerse the pH probe into the solution being tested, ensuring that the probe is fully submerged.
c. Allow the pH reading to stabilize on the pH meter display, and record the pH value.
- pH Indicator Strips or Papers: pH indicator strips or papers are simple and inexpensive tools for measuring pH. These strips are impregnated with a mixture of pH-sensitive compounds that change color in response to different pH levels. You dip the strip into the solution being tested, and the color change corresponds to a specific pH value. You then match the strip's color to a provided color chart to determine the pH value.
- pH Indicator Solutions: pH indicator solutions are liquid formulations that change color depending on the pH of the solution being tested. They work similarly to indicator strips but are in liquid form. You add a few drops of the indicator solution to the solution being tested, and the resulting color indicates the pH value. pH indicator solutions often come with a color chart for reference.
It's important to note that the accuracy and precision of pH measurements may vary depending on the method and the quality of the equipment or indicators used. For critical applications, such as scientific research or industrial processes, it is recommended to use calibrated pH meters for the most accurate results.
What is a pH meter?
A pH meter is a scientific instrument used to measure the acidity or alkalinity of a solution. It consists of a pH probe (also known as a pH electrode) and a pH meter device.
The pH probe is the key component of a pH meter. It is a specialized electrode that contains a glass membrane and a reference electrode. The glass membrane is sensitive to hydrogen ion (H+) concentration changes in the solution. When the probe is immersed in a solution, an electrical potential is generated between the glass membrane and the reference electrode, which is proportional to the hydrogen ion activity in the solution.
The pH meter device is a handheld or benchtop instrument that connects to the pH probe. It reads the electrical potential generated by the pH probe and converts it into a pH value. Modern pH meters often have digital displays, calibration functions, and other features to provide accurate and convenient pH measurements.
To measure pH using a pH meter, the following steps are typically followed:
- Calibration: Before use, the pH meter needs to be calibrated using standard buffer solutions with known pH values. This ensures the accuracy of the pH measurements. Typically, two or more buffer solutions are used for calibration, and the pH meter is adjusted based on the readings obtained.
- Preparation: The pH probe is rinsed with distilled water and then immersed in the solution being tested. It is important to handle the pH probe carefully to avoid damage or contamination.
- Measurement: Once the pH probe is immersed in the solution, the pH meter device is switched on, and the reading stabilizes over a few seconds. The pH value is displayed on the meter's screen, representing the acidity or alkalinity of the solution.
pH meters are widely used in various scientific, industrial, and research applications where accurate pH measurements are required. They offer higher precision and accuracy compared to pH indicator papers or strips, making them suitable for critical experiments, quality control processes, and environmental monitoring.

Fig 2. Digital Ph Meter
How does the pH meter work?
A pH meter works based on the principle of measuring the electrical potential difference generated by a pH-sensitive electrode, also known as a pH probe or pH electrode. Here's a step-by-step explanation of how a pH meter works:
- pH Probe Construction: The pH probe consists of two main components: a glass electrode and a reference electrode. The glass electrode is the pH-sensitive part and is usually a thin, bulb-shaped membrane made of special glass. This glass membrane is selective to hydrogen ions (H+) and allows them to pass through it. The reference electrode is usually a silver-silver chloride electrode immersed in a potassium chloride (KCl) electrolyte solution. It provides a stable reference potential against which the pH-sensitive glass electrode can be measured.
- Electrode Immersion: The pH probe is immersed in the solution being tested. The glass membrane of the pH electrode comes into contact with the solution, while the reference electrode remains in contact with the electrolyte solution inside the pH probe.
- Ion Exchange: When the pH probe is immersed in the solution, hydrogen ions (H+) from the solution interact with the glass membrane of the pH electrode. The glass membrane allows the exchange of hydrogen ions with the internal solution inside the pH probe, causing a change in electrical potential.
- Potential Difference: The exchange of hydrogen ions generates an electrical potential difference between the glass electrode (sensitive to H+ ions) and the reference electrode. This potential difference is proportional to the concentration of hydrogen ions in the solution and reflects its pH level.
- Measurement: The pH meter device is connected to the pH probe and measures the electrical potential difference between the glass electrode and the reference electrode. The pH meter device typically contains a high-input impedance amplifier that converts the electrical potential into a pH value. The pH value is then displayed on the screen of the pH meter device.
- Calibration: To ensure accuracy, pH meters need to be calibrated using standard buffer solutions with known pH values. The calibration process adjusts the pH meter to accurately measure the potential difference generated by the pH probe at different pH values.
By measuring the electrical potential difference between the pH-sensitive glass electrode and the reference electrode, a pH meter provides a precise and quantitative measurement of the acidity or alkalinity of a solution.
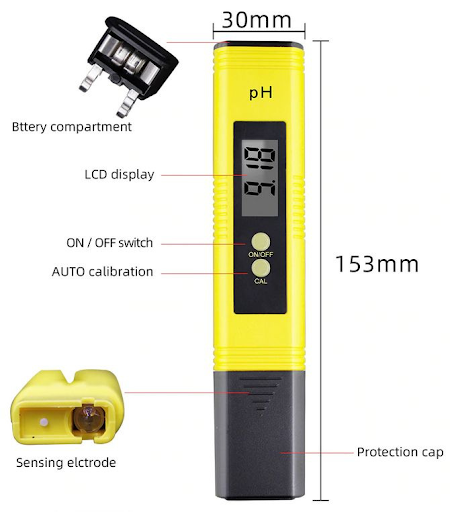
Fig 3. pH meter structure
pH meter formulas
pH meters do not involve specific formulas in the traditional sense. However, there are a few important concepts and calculations related to pH measurements that are useful to understand. Here are three key aspects:
- pH Calculation:
- pH is calculated using the formula:
pH = -log[H+], where [H+]
represents the hydrogen ion concentration in moles per liter.
- Alternatively, pH can be determined using the formula: pH = 14 - pOH, where pOH is the negative logarithm of the hydroxide ion (OH-) concentration.
- These formulas allow you to convert the concentration of hydrogen ions or hydroxide ions into a pH value.
- Nernst Equation:
- The Nernst equation is used to relate the measured electrical potential of a pH electrode to the pH of the solution being tested. It is a fundamental equation in electrochemistry.
- The Nernst equation for a pH electrode is
E = E₀ + (0.0592/n) * log[H+]
where E is the measured potential, E₀ is the standard electrode potential, n is the number of electrons involved in the redox reaction (usually 1 for pH electrodes), and [H+] is the hydrogen ion concentration.
- The Nernst equation allows the pH meter to convert the electrical potential measured by the pH probe into a pH value.
- Calibration:
- pH meters need to be calibrated using buffer solutions with known pH values. The calibration process adjusts the pH meter to accurately measure the potential difference generated by the pH probe at different pH values.
- Typically, two or more buffer solutions are used for calibration, such as pH 4.0, pH 7.0, and pH 10.0. The pH meter is adjusted based on the measured potential difference at these known pH values.
It's worth noting that the specific implementation and internal calculations of pH meters may vary among different models and manufacturers. The formulas and equations mentioned here provide a general understanding of pH measurements and how pH meters work.
An example of using the formula
Here's an example that demonstrates the use of the pH formula to calculate the pH value of a solution:
Example: Calculate the pH of a solution with a hydrogen ion concentration of 1.0 x 10^-4 M.
Using the formula pH = -log[H+], we can substitute the given hydrogen ion concentration into the equation:
pH = -log(1.0 x 10^-4)
To evaluate this mathematically, we take the logarithm of 1.0 x 10^-4 and then multiply it by -1:
pH = -(-4) = 4
Therefore, the pH of the solution with a hydrogen ion concentration of 1.0 x 10^-4 M is 4.
This calculation illustrates how the pH formula allows us to determine the pH value of a solution based on its hydrogen ion concentration. Remember that pH is a logarithmic scale, so each change of one unit in pH represents a tenfold change in hydrogen ion concentration.
Table 1. Formulas table
| Formula | Description |
| pH = -log[H+] | Calculates pH based on hydrogen ion concentration. |
| pH = 14 - pOH | Calculates pH based on pOH (negative logarithm of hydroxide ion concentration). |
| Nernst Equation: E = E₀ + (0.0592/n) * log[H+] | Relates the measured potential of a pH electrode to pH, taking into account the standard electrode potential, number of electrons involved (n), and hydrogen ion concentration ([H+]). |
| pOH = -log[OH-] | Calculates pOH based on hydroxide ion concentration. |
| [H+] = 10^(-pH) | Calculates hydrogen ion concentration based on pH. |
| [OH-] = 10^(-pOH) | Calculates hydroxide ion concentration based on pOH. |
| pH = pKa + log([A-]/[HA]) | Henderson-Hasselbalch equation, relates pH, pKa (acid dissociation constant), and the ratio of conjugate base ([A-]) to weak acid ([HA]) concentrations. |
| [H+] = 10^(-pH) and [OH-] = 10^(-pOH) | In neutral water, [H+] equals [OH-], enabling the calculation of one from the other. |
These formulas and equations are commonly used in pH calculations and in understanding the relationships between pH, hydrogen ion concentration, and other related parameters.
What is the pH reading in the pH meter?
In a pH meter, the pH reading refers to the numerical value displayed on the pH meter device that represents the acidity or alkalinity of a solution. It indicates the pH level of the solution being measured.
The pH reading is typically a decimal number displayed on the pH meter's screen, ranging from 0 to 14, with 7 being considered neutral. pH values below 7 indicate acidity, with lower numbers indicating stronger acidity. pH values above 7 indicate alkalinity (basicity), with higher numbers indicating stronger alkalinity.
For example, a pH reading of 4 indicates an acidic solution, while a pH reading of 10 indicates an alkaline solution. The specific pH reading allows for quantifying the relative concentration of hydrogen ions (H+) in the solution, which directly affects its pH level.
It's important to note that pH readings can vary depending on the calibration of the pH meter, the quality and maintenance of the pH probe, and the accuracy of the measurement technique. Proper calibration and handling of the pH meter are essential to obtain accurate and reliable pH readings.

Fig 4. A pH meter
How important is the pH meter?
pH meters are highly important and widely used instruments in various fields for several reasons:
- Accurate pH Measurement: pH meters provide precise and quantitative measurements of the acidity or alkalinity of a solution. They offer higher accuracy compared to other methods, such as pH indicator papers or strips. This accuracy is particularly crucial in scientific research, quality control processes, environmental monitoring, and many other applications where precise pH measurements are essential.
- Wide Range of Applications: pH measurements are relevant in numerous fields, including chemistry, biology, environmental science, agriculture, food and beverage industry, pharmaceuticals, water treatment, and more. pH meters enable researchers, technicians, and professionals in these fields to monitor and control pH levels, assess the effectiveness of processes, troubleshoot problems, and ensure product quality and safety.
- Versatility: pH meters can measure pH in a wide range of solutions, from aqueous solutions to complex biological samples. They can be used in both laboratory settings and field applications, making them versatile instruments for pH analysis in various environments.
- Calibration and Standardization: pH meters require regular calibration using buffer solutions with known pH values. This calibration process ensures the accuracy of pH measurements by adjusting the pH meter's readings based on the known standards. Standardization of pH measurements allows for consistent and reliable comparison of results across different laboratories and settings.
- Efficiency and Time-saving: pH meters provide real-time pH measurements, allowing for immediate analysis and decision-making. They eliminate the need for time-consuming and subjective color comparisons involved in traditional pH indicator methods. This efficiency is particularly valuable in time-sensitive processes or when large numbers of samples need to be analyzed.
- Documentation and Data Logging: Many modern pH meters come with advanced features such as data logging capabilities. They allow for the recording and storage of pH measurements along with additional information like time, date, sample details, and user annotations. This feature facilitates data management, analysis, and traceability.
In summary, pH meters play a critical role in accurately measuring and monitoring pH levels across a wide range of applications. They provide reliable results, streamline workflows, and contribute to the understanding, control, and optimization of processes in various scientific, industrial, and environmental contexts.
What are the types of pH meters?
There are several types of pH meters available, each with its own design, functionality, and application. Here are some common types of pH meters:
- Portable pH Meters: These pH meters are compact, handheld devices designed for field measurements. They are battery-operated, easy to carry, and often have built-in features such as waterproofing and rugged construction for durability in outdoor or challenging environments. Portable pH meters are commonly used in environmental monitoring, agriculture, and water quality testing.
- Benchtop pH Meters: Benchtop pH meters are larger, more advanced instruments typically used in laboratory settings. They offer higher accuracy, precision, and advanced features compared to portable meters. Benchtop pH meters often have larger displays, more extensive data storage, and advanced calibration options. They may also have additional measurement capabilities, such as temperature compensation. These pH meters are widely used in scientific research, quality control labs, and educational institutions.
- Pen-Style pH Meters: Pen-style pH meters are compact and pen-shaped, resembling a writing instrument. They are easy to use, portable, and suitable for quick pH measurements. Pen-style pH meters are commonly used for general pH testing in various applications, including aquariums, pools, hydroponics, and home use.
- Combination Meters: Combination meters, also known as multi-parameter meters, are versatile instruments that can measure pH along with other parameters such as temperature, conductivity, dissolved oxygen, or ion concentration. These meters offer the convenience of measuring multiple parameters with a single device, reducing the need for multiple instruments. Combination meters are often used in environmental monitoring, water analysis, and industrial applications.
- In-Line pH Meters: In-line pH meters are designed to be installed directly in a process or pipeline to continuously monitor pH levels. They are integrated into the flow path of a liquid stream and provide real-time pH measurements. In-line pH meters are commonly used in industrial processes, wastewater treatment plants, and manufacturing operations.
It's important to consider the specific requirements of your application and choose the appropriate type of pH meter that suits your needs in terms of accuracy, portability, functionality, and budget.
Here's a detailed explanation of each type of pH meter, including its working principle, applications, pH range, usage, as well as the pros and cons associated with each type:
Portable pH Meters:
- Working Principle: Portable pH meters operate based on the same principles as other pH meters. They use a pH-sensitive glass electrode and a reference electrode to measure the electrical potential generated by the solution being tested. The pH meter device converts the potential into a pH reading.
- Applications: Portable pH meters are commonly used for field measurements in environmental monitoring, agriculture, water quality testing, and other applications where on-site pH analysis is required.
- pH Range: Typically, portable pH meters cover the pH range from 0 to 14.
- Usage: Portable pH meters are battery-operated and designed for ease of use and portability. They are suitable for outdoor use, offering features like waterproofing and rugged construction.
- Pros: Portable, suitable for field measurements, durable, and convenient for on-site analysis.
- Cons: May have slightly lower accuracy and precision compared to benchtop models, and limited advanced features.

Fig 5. Portable PH meter
-
Benchtop pH Meters
- Working Principle: Benchtop pH meters operate using the same principles as other pH meters. They consist of a pH electrode and a reference electrode, which measure the electrical potential of the solution to determine pH.
- Applications: Benchtop pH meters are widely used in scientific research, quality control labs, educational institutions, and other laboratory settings where high accuracy and advanced features are required.
- pH Range: Benchtop pH meters cover the full pH range from 0 to 14.
- Usage: Benchtop pH meters are larger and more advanced than portable models. They offer higher accuracy, precision, and advanced features like data storage, advanced calibration options, and temperature compensation.
- Pros: High accuracy, precision, advanced features, suitable for laboratory use, customizable settings.
- Cons: Bulkier and less portable compared to portable models.

Fig 6. Benchtop pH Meters
-
Pen-Style pH Meters
- Working Principle: Pen-style pH meters use a pH electrode and a reference electrode to measure the electrical potential of the solution and convert it into a pH reading.
- Applications: Pen-style pH meters are commonly used for quick and general pH testing in various applications such as aquariums, pools, hydroponics, and home use.
- pH Range: Pen-style pH meters cover the pH range from 0 to 14.
- Usage: Pen-style pH meters are compact and easy to use. They are suitable for quick pH measurements, providing convenience and portability.
- Pros: Portable, easy to use, convenient for quick pH measurements.
- Cons: May have slightly lower accuracy and precision compared to more advanced models, limited features, and customization options.

Fig 7. Pen-style pH meter
-
Combination Meters
- Working Principle: Combination meters operate on the same principles as other pH meters, but they also include additional measurement capabilities for parameters such as temperature, conductivity, dissolved oxygen, or ion concentration.
- Applications: Combination meters are versatile instruments used in environmental monitoring, water analysis, industrial processes, and other applications where multiple parameters need to be measured simultaneously.
- pH Range: Combination meters cover the full pH range from 0 to 14.
- Usage: Combination meters provide the convenience of measuring multiple parameters with a single device, reducing the need for multiple instruments. They offer flexibility and efficiency in multi-parameter analysis.
- Pros: Multi-parameter capability, versatility, convenience, reduced equipment requirements.
- Cons: May have a slightly higher cost compared to single-parameter meters, and potential complexity in the interpretation of multiple parameter measurements.
Fig 8. CONSTRUCTION DIAGRAM of Combination Meters
-
In-Line pH Meters
- Working Principle: In-line pH meters are installed directly in a process or pipeline to continuously monitor pH levels. They employ a pH electrode and reference electrode immersed in the flowing liquid, providing real-time pH measurements.
- Applications: In-line pH meters are used in industrial processes, wastewater treatment plants, manufacturing operations, and other applications where continuous pH monitoring is necessary.
- pH Range: In-line pH meters cover the full pH range from 0 to 14.
- Usage: In-line pH meters are integrated into the flow path of the liquid stream and provide continuous, real-time pH measurements, allowing for process control and optimization.
- Pros: Continuous monitoring, real-time measurements, suitable for process control.
- Cons: May require installation and integration into the process, potential maintenance and calibration challenges, and higher cost compared to other types of pH meters.

Fig 9. pH prob
It's important to consider the specific requirements of your application and choose the pH meter type that best fits your needs in terms of accuracy, portability, functionality, and budget.
Table 2. Comparing pH meter types table
| Type | Working Principle | Applications | pH Range | Usage | Pros | Cons |
| Portable pH Meters | pH electrode and reference electrode measure the electrical potential of the solution | Field measurements, environmental monitoring, agriculture, water quality testing | 0-14 | Portable, battery-operated, suitable for outdoor use | Portable, durable, convenient for on-site analysis | Slightly lower accuracy and advanced features compared to benchtop meters |
| Benchtop pH Meters | pH electrode and reference electrode measure the electrical potential of the solution | Scientific research, quality control labs, educational institutions | 0-14 | Laboratory use, higher accuracy, advanced features | High accuracy, precision, advanced features | Bulkier and less portable compared to portable meters |
| Pen-Style pH Meters | pH electrode and reference electrode measure the electrical potential of the solution | Quick pH testing in various applications (e.g., aquariums, pools) | 0-14 | Compact, easy to use, portable | Portable, easy to use | Slightly lower accuracy and precision compared to advanced models |
| Combination Meters | pH electrode and reference electrode measure the electrical potential of the solution along with additional measurement capabilities (e.g., temperature, conductivity) | Environmental monitoring, water analysis, industrial processes | 0-14 | Multi-parameter measurement with a single device | Multi-parameter capability, versatility | Slightly higher cost compared to single-parameter meters |
| In-Line pH Meters | pH electrode and reference electrode continuously measure the electrical potential of the solution | Industrial processes, wastewater treatment plants, manufacturing operations | 0-14 | Continuous monitoring, integrated into the process | Continuous monitoring, real-time measurements | Installation and integration required, potential maintenance and calibration challenges, higher cost |
It's important to note that this table provides a general overview of the characteristics and pros/cons of each pH meter type. The specific features, performance, and limitations of individual models may vary among different manufacturers and models.
Where is the pH meter used?
pH meters are used in various fields and industries where pH measurement is important. Some common areas of application for pH meters include:
- Chemistry and Chemical Analysis: pH meters are extensively used in chemical laboratories for accurate pH measurements of solutions and substances. They are crucial in determining the acidity or alkalinity of chemical solutions and assessing the progress of chemical reactions.
- Environmental Monitoring: pH meters play a significant role in environmental monitoring, particularly in water quality assessment. They are used to measure the pH levels of natural water bodies, such as lakes, rivers, and oceans, to evaluate their ecological health and detect any potential pollution or environmental changes.
- Agriculture and Soil Science: pH meters are utilized in agriculture to measure the pH of soil and irrigation water. The pH of soil affects the availability of nutrients to plants, and maintaining an optimal pH range is crucial for crop growth and productivity. pH meters help farmers and researchers assess soil acidity or alkalinity and make informed decisions about soil amendments and fertilizers.
- Food and Beverage Industry: pH meters are used in food processing and quality control to monitor and control the pH levels of various food and beverage products. pH is a critical factor in food safety, preservation, and flavor development. pH measurements are employed in the production of dairy products, beverages, canned foods, and many other food items.
- Pharmaceuticals and Biotechnology: pH monitoring is essential in pharmaceutical research, drug formulation, and biotechnological processes. pH meters are utilized to optimize and control the pH conditions in fermentation processes, cell culture media, and drug formulations, ensuring the stability and efficacy of pharmaceutical products.
- Water Treatment and Wastewater Management: pH meters are integral to water treatment plants and wastewater management facilities. They are used to monitor and control the pH levels in water treatment processes, such as coagulation, flocculation, and disinfection. pH measurements assist in ensuring proper disinfection, minimizing corrosion, and maintaining regulatory compliance.
- Aquaculture and Aquariums: pH meters are employed in aquaculture facilities and aquariums to monitor and maintain optimal pH conditions for aquatic organisms. pH levels significantly impact the health and survival of fish, crustaceans, and other aquatic species. pH meters aid in maintaining suitable pH ranges for aquatic environments.
These are just a few examples of the many areas where pH meters find application. pH measurement is essential in numerous scientific, industrial, and research fields that require accurate monitoring and control of pH levels for optimal outcomes, safety, and quality.

Fig 10. pH meter usage
What is the pH meter used for?
pH meters are used for measuring the acidity or alkalinity of a solution, which is quantified by its pH value. Here are some specific uses of pH meters:
- pH Measurement: The primary purpose of pH meters is to measure the pH level of a solution accurately. pH measurements help in assessing the acidity or alkalinity of various substances, liquids, and materials.
- Quality Control: pH meters are extensively used in quality control processes across different industries. They help ensure that products meet desired pH specifications and maintain consistent quality. For example, in the food and beverage industry, pH meters are used to monitor and control the pH of products such as juices, dairy products, and canned goods.
- Chemical Analysis: pH meters are indispensable tools in chemical laboratories. They aid in analyzing and characterizing chemical solutions, determining the endpoint of titrations, monitoring pH changes during chemical reactions, and assessing the acidity or basicity of compounds.
- Environmental Monitoring: pH meters are widely used in environmental monitoring programs, particularly in water quality assessment. They help measure the pH of natural water bodies such as rivers, lakes, and oceans to evaluate the health of aquatic ecosystems, detect pollution, and assess the impact of industrial activities or agricultural runoff.
- Agriculture and Soil Science: pH meters are essential for measuring the pH of soil and irrigation water in agriculture and soil science. They provide valuable information about soil acidity or alkalinity, which directly affects nutrient availability and plant growth. pH measurements help farmers and researchers make informed decisions about soil amendments and fertilizers to optimize crop productivity.
- Water Treatment: pH meters play a crucial role in water treatment plants. They are used to monitor and control the pH levels during various water treatment processes like coagulation, flocculation, disinfection, and pH adjustment. Maintaining the appropriate pH range ensures effective treatment, prevents corrosion, and ensures compliance with water quality regulations.
- Aquaculture and Aquariums: pH meters are used in aquaculture facilities and aquariums to monitor and control the pH levels of water. Maintaining the optimal pH range is crucial for the health and survival of aquatic organisms, as it affects their physiology, growth, and overall well-being.
These are just a few examples of the diverse applications of pH meters. pH measurements provide valuable insights in numerous fields, aiding in research, process control, product development, and ensuring optimal conditions for various applications.

Fig 11. pH meter in labratory
Installation and maintenance
How can use the pH meter?
Using a pH meter typically involves the following steps:
- Calibrating the pH Meter:
- Prepare calibration solutions: Obtain at least two pH calibration solutions that span the pH range of your expected measurements (e.g., pH 4 and pH 7 solutions).
- Rinse the electrode: Rinse the pH electrode with distilled water to remove any contaminants.
- Immerse the electrode: Immerse the pH electrode into the first calibration solution and wait for the reading to stabilize.
- Adjust calibration: Follow the manufacturer's instructions to adjust the pH meter to the known pH value of the calibration solution. This step may involve pressing specific buttons or adjusting trimmers on the pH meter.
- Repeat calibration: Repeat the calibration process with the second calibration solution (e.g., pH 7) to ensure accuracy.
- Preparing the Sample:
- Ensure cleanliness: Ensure that the electrode and any containers or tools that will come in contact with the sample are clean and free from contaminants.
- Sample preparation: Depending on the application, you may need to prepare the sample by diluting, filtering, or adjusting the temperature.
- Taking pH Measurements:
- Rinse the electrode: Rinse the pH electrode with distilled water to remove any residual traces from the previous measurement.
- Immerse the electrode: Immerse the pH electrode into the sample solution, ensuring that it is fully submerged.
- Wait for stabilization: Allow the pH reading to stabilize on the pH meter display. This typically takes a few seconds or until the reading becomes constant.
- Record the pH reading: Once the reading has stabilized, record the pH value displayed on the meter.
- Cleaning and Maintenance:
- Rinse the electrode: After each measurement, rinse the pH electrode with distilled water to remove any residues.
- Store properly: Store the pH meter and electrode according to the manufacturer's instructions, usually in a storage solution or protective cap to keep the electrode hydrated and prevent damage.
It's important to consult the specific instructions provided by the manufacturer of your pH meter, as the steps may vary slightly depending on the model. Additionally, make sure to follow any safety precautions and guidelines provided by the manufacturer to ensure accurate and safe usage of the pH meter.
pH meter calibration
Calibration is a crucial step in using a pH meter to ensure accurate and reliable pH measurements. Here's a general guide on how to calibrate a pH meter:
- Gather Calibration Solutions:
- Obtain at least two pH calibration solutions that cover the pH range of your expected measurements. Commonly used calibration solutions include pH 4, pH 7, and pH 10. The pH calibration solutions should be fresh, not expired, and stored properly according to the manufacturer's instructions.
- Rinse the pH Electrode:
- Rinse the pH electrode with distilled water to remove any contaminants or residues from the previous measurement. Gently blot or shake off excess water.
- Perform Calibration:
- Turn on the pH meter and let it warm up, if required, according to the manufacturer's instructions.
- Immerse the pH electrode in the first calibration solution. Ensure the electrode is fully submerged and not touching the container walls or the bottom.
- Stir gently or wait for the reading to stabilize as per the manufacturer's recommended time.
- Adjust the pH meter: Use the calibration controls on the pH meter to adjust the reading to match the known pH value of the calibration solution. The specific adjustment process may vary depending on the pH meter model. Follow the manufacturer's instructions to perform the adjustment accurately.
- Rinse the electrode with distilled water and blot or shake off excess water before moving to the next calibration solution.
- Repeat the calibration process with the second calibration solution. This helps verify the accuracy of the pH meter and accounts for any potential calibration drift.
- Some pH meters allow for multi-point calibration, which involves additional calibration points with intermediate pH values. Follow the manufacturer's instructions if your pH meter supports multi-point calibration.
- Verify Calibration:
- After calibration, check the readings in calibration solutions to ensure they are within an acceptable range. If the readings are significantly off, repeat the calibration process or consult the pH meter's manual for troubleshooting guidance.
- Record Calibration:
- Record the calibration details, including the date, time, pH values of the calibration solutions, and any adjustments made. This information helps maintain a record of calibration history for future reference.
- Regular Calibration:
- Regularly calibrate the pH meter to maintain accuracy. The frequency of calibration depends on the manufacturer's recommendations, frequency of use, and the nature of the samples being tested. For most applications, calibration before each use or at least once a day is recommended.
It's important to note that the specific calibration procedure may vary slightly depending on the pH meter model and manufacturer. Always refer to the user manual and follow the instructions provided by the pH meter manufacturer for accurate calibration.
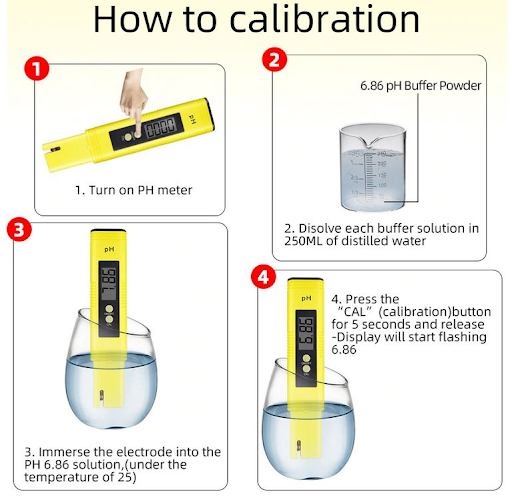
Fig 12. How to calibrate a pH meter
pH meter accuracy
The accuracy of a pH meter refers to how closely it measures the true pH value of a solution. It is important to have an accurate pH meter to obtain reliable and precise pH measurements. The accuracy of a pH meter can be affected by several factors:
- Calibration: Proper calibration is essential for accurate pH measurements. Regular calibration using fresh calibration solutions is necessary to ensure accurate readings. Calibrating the pH meter at multiple points across the pH range helps improve accuracy and correct for any potential deviations.
- Electrode Condition: The condition of the pH electrode can impact accuracy. The electrode should be clean and free from any contaminants or residues. Regularly rinsing the electrode with distilled water and storing it properly when not in use helps maintain its performance.
- Temperature Compensation: pH measurements are temperature-dependent, and pH meters typically have automatic or manual temperature compensation features. Temperature fluctuations can affect pH readings, so using accurate temperature compensation ensures more accurate results, especially for samples with varying temperatures.
- Electrode Age and Maintenance: Over time, pH electrodes can degrade and lose accuracy. Regular maintenance, such as cleaning, recalibration, and electrode replacement when necessary, helps maintain accuracy.
- Sample Interference: Some samples may contain substances that can interfere with pH measurements, causing inaccuracies. It is important to consider potential interferences and use appropriate sample preparation techniques or specialized electrodes to mitigate their impact.
- Quality of pH Standards: The accuracy of pH calibration solutions used for calibration affects the accuracy of the pH meter. Using high-quality, fresh calibration standards with known pH values helps ensure more accurate calibration and subsequent pH measurements.
It's important to note that pH meters have an associated accuracy specification provided by the manufacturer. This specification indicates the maximum permissible error or deviation from the true pH value. The accuracy of pH meters can vary depending on the model, quality, and calibration practices. It is recommended to choose a pH meter from a reputable manufacturer and follow their guidelines for calibration and maintenance to achieve the best possible accuracy.
Safety tips while using a pH meter
While using a pH meter, it's important to follow safety guidelines to ensure your personal safety and to handle the equipment properly. Here are some safety tips to consider:
- Personal Protective Equipment (PPE): Wear appropriate PPE, such as gloves and safety goggles, especially when handling corrosive or hazardous samples that may come in contact with your skin or eyes.
- Read the User Manual: Familiarize yourself with the specific safety instructions and guidelines provided by the manufacturer in the user manual or accompanying documentation. Follow them carefully to ensure safe and proper usage of the pH meter.
- Chemical Handling: Be cautious when handling chemicals, especially corrosive substances or strong acids/bases used for calibration or sample preparation. Follow proper chemical handling procedures, use appropriate containment measures, and work in a well-ventilated area or under a fume hood if necessary.
- Avoid Contamination: Avoid cross-contamination between samples. Rinse the electrode with distilled water and blot or shake off excess water before moving from one sample to another. This prevents potential contamination and ensures accurate measurements.
- Electrode Handling: Handle the pH electrode with care to prevent damage. Avoid touching the glass bulb of the electrode with bare hands or rough surfaces. Use the electrode holder or appropriate tools when handling or inserting/removing the electrode.
- Proper Storage: Store the pH meter and electrode according to the manufacturer's instructions. Typically, pH electrodes need to be stored in a storage solution or a moist environment to keep them hydrated and maintain their performance. Avoid storing the electrode in extreme temperatures or exposing it to direct sunlight.
- Electrical Safety: Handle the pH meter and its power source (batteries or power cord) with caution. Avoid exposing the equipment to water or liquids that could cause electrical hazards. Unplug the pH meter from the power source when not in use.
- Maintenance and Calibration: Regularly maintain and calibrate the pH meter as per the manufacturer's recommendations. This ensures accurate readings and helps identify any issues or deviations early on.
- Disposal of Solutions: Follow proper procedures for the disposal of calibration solutions, samples, and any chemicals used during pH measurements. Adhere to local regulations and guidelines for safe disposal.
- Training and Knowledge: Ensure that you have received proper training on the safe operation of the pH meter and an understanding of the potential hazards associated with the samples and chemicals you are working with. Seek guidance from experienced personnel or consult with experts if needed.
Remember to prioritize safety at all times when using a pH meter. If you encounter any safety concerns or difficulties, consult the manufacturer's guidelines or seek assistance from relevant experts or professionals.
Pros and Cons of using pH meter
Pros of using a pH meter:
- Accuracy: pH meters provide precise and accurate measurements of the acidity or alkalinity of a solution, allowing for precise control and analysis in various applications.
- Speed: pH meters provide quick and real-time pH readings, enabling fast decision-making and immediate adjustments if necessary.
- Wide Range: pH meters can measure a wide range of pH values, accommodating diverse samples and applications.
- Versatility: pH meters can be used in various industries and fields, including research, environmental monitoring, food and beverage production, agriculture, water treatment, and more.
- User-Friendly: Modern pH meters are designed with user-friendly interfaces, intuitive controls, and clear displays, making them easy to operate and interpret the results.
- Temperature Compensation: Many pH meters come with automatic temperature compensation (ATC), which adjusts the pH readings based on the sample temperature, ensuring more accurate results.
- Data Logging: Advanced pH meters may have data logging capabilities, allowing you to store and retrieve pH measurements for analysis, documentation, and quality control purposes.
Cons of using a pH meter:
- Cost: pH meters can be relatively expensive, especially for high-quality and advanced models, which may pose a financial constraint for some users.
- Maintenance: pH meters require regular maintenance, including calibration, electrode cleaning, and storage in proper solutions. Failure to perform maintenance can lead to inaccurate readings and reduced lifespan of the equipment.
- Fragility: pH electrodes, particularly glass electrodes, can be fragile and susceptible to damage if mishandled. Careful handling and proper storage are necessary to maintain their accuracy and durability.
- Electrode Degradation: Over time, pH electrodes can degrade, leading to decreased accuracy and performance. Replacing electrodes periodically may be required, adding to the overall cost of ownership.
- Sample Contamination: If proper precautions are not taken, samples can contaminate the pH electrode, affecting accuracy and leading to cross-contamination between samples.
- Calibration Requirements: pH meters need regular calibration using standard solutions to ensure accuracy. This requires access to calibration solutions and the time and effort to perform the calibration procedure.
- Limitations for Non-Aqueous Samples: pH meters are primarily designed for measuring the pH of aqueous solutions. They may not provide accurate readings for non-aqueous samples without specialized equipment or electrodes.
How can choose the right pH meter?
Choosing the right pH meter involves considering several factors to ensure it meets your specific requirements. Here are some key considerations to help you select the appropriate pH meter:
- Accuracy and Precision: Look for a pH meter with a high level of accuracy and precision. Check the manufacturer's specifications for the meter's accuracy, expressed as the maximum permissible error or deviation from the true pH value. Consider your application's requirements and choose a pH meter that provides the necessary level of accuracy for your measurements.
- Measurement Range: Determine the pH range of the samples you will be measuring. pH meters come with different measurement ranges, so select a meter that covers the pH range you typically work with. Ensure the pH meter has a range wide enough to accommodate your specific needs.
- Resolution: The resolution of a pH meter refers to the smallest increment it can display. A higher resolution allows for more precise pH readings. Consider the level of resolution required for your application and choose a pH meter with a suitable resolution.
- Calibration: Check the calibration requirements of the pH meter. Some meters may require single-point calibration, while others may support multi-point calibration. Consider the ease of calibration and the availability of compatible calibration solutions.
- Temperature Compensation: pH measurements can be affected by temperature variations. pH meters with automatic temperature compensation (ATC) adjust the pH readings based on the sample temperature, providing more accurate results. Consider whether ATC is necessary for your measurements, especially if you work with samples at different temperatures.
- Electrode Type:pH meters use different types of electrodes, such as glass, combination (pH and reference electrode in one), or specialty electrodes for specific applications. Choose an electrode type that is suitable for your samples and provides accurate and consistent readings.
- Durability and Build Quality: Consider the build quality and durability of the pH meter. Look for robust construction, resistant materials, and waterproof or splash-proof features if needed for your application. A sturdy pH meter can withstand regular use and potentially harsh environments.
- User-Friendly Features: Evaluate the user interface and ease of use of the pH meter. Consider features such as a clear and readable display, intuitive controls, and ergonomic design for comfortable handling. Some meters may also offer additional features like data logging, automatic shut-off, or connectivity options for data transfer.
- Budget: Determine your budget for a pH meter and consider the price range of available options. It's important to strike a balance between affordability and meeting your specific needs for accuracy and functionality.
- Reviews and Recommendations: Read reviews and seek recommendations from trusted sources, such as scientific publications, colleagues, or online forums, to gain insights into the performance and reliability of different pH meter models and brands.
By carefully considering these factors, you can select a pH meter that aligns with your requirements, ensuring accurate and reliable pH measurements for your specific application.
Top brands of pH meter
Several reputable brands offer high-quality pH meters. Here are some top brands known for their pH meters:
- Hanna Instruments: Hanna Instruments is a well-established brand known for its wide range of analytical instruments, including pH meters. They offer a variety of pH meters suitable for various applications, ranging from basic handheld meters to advanced benchtop models.
- Thermo Fisher Scientific: Thermo Fisher Scientific is a leading provider of scientific instruments and laboratory equipment. They offer pH meters under their brand names such as Orion and Thermo Scientific. Their pH meters are known for their accuracy, durability, and advanced features.
- Mettler Toledo: Mettler Toledo is a renowned manufacturer of precision instruments, including pH meters. Their pH meters are known for their high accuracy, reliability, and user-friendly interfaces. They offer a range of portable, benchtop, and in-line pH meters to cater to different needs.
- Ohaus: Ohaus is a trusted brand that offers a wide range of laboratory equipment, including pH meters. Their pH meters are known for their accuracy, durability, and user-friendly operation. Ohaus provides both portable and benchtop models suitable for various applications.
- Oakton Instruments: Oakton Instruments specializes in the manufacturing of scientific instruments and meters, including pH meters. They offer a range of pH meters with different features and functionalities, catering to diverse laboratory and field applications.
- Eutech Instruments: Eutech Instruments is a reputable brand that focuses on water quality analysis and offers a wide range of pH meters. Their pH meters are known for their accuracy, reliability, and robust construction. They provide pH meters suitable for various industries, including environmental, pharmaceutical, and research laboratories.
- Apera Instruments: Apera Instruments is known for its affordable yet reliable pH meters. They offer a range of pH meters designed for different applications, including handheld and benchtop models. Apera Instruments' pH meters are popular among professionals and hobbyists alike.
It's important to note that the suitability of a brand or specific pH meter model may vary depending on your specific requirements, application, and budget. It's recommended to research and read reviews to determine which brand and model best meet your needs.
Conclusion
In conclusion, pH meters are essential tools for measuring the acidity or alkalinity of a solution. They provide accurate and reliable pH readings, making them valuable in various industries and applications such as environmental monitoring, water quality analysis, research laboratories, food and beverage production, and more.
pH meters work based on the principle of measuring the potential difference between the reference electrode and the pH-sensitive glass electrode. This potential difference is converted into a pH value using the Nernst equation.
There are different types of pH meters available, including portable handheld meters and more advanced benchtop models. Each type has its working principle, advantages, and limitations. Factors such as accuracy, measurement range, resolution, calibration requirements, temperature compensation, and durability should be considered when choosing the right pH meter for your needs.
When using a pH meter, it is crucial to follow safety guidelines, including wearing appropriate personal protective equipment (PPE) and handling chemicals with care. Regular calibration, maintenance, and proper storage of the pH meter and electrode are important for accurate and reliable measurements.
Some of the top brands known for their pH meters include Hanna Instruments, Thermo Fisher Scientific, Mettler Toledo, Ohaus, Oakton Instruments, Eutech Instruments, and Apera Instruments. These brands offer a range of pH meters with varying features, accuracy levels, and price points.
By selecting the right pH meter and following proper procedures, users can obtain accurate pH measurements, monitor changes in acidity or alkalinity, and make informed decisions in their respective fields of application.
To recap
- What is pH and why is it important?
- pH is a measure of the acidity or alkalinity of a solution. It is important because pH influences chemical reactions, biological processes, and the behavior of various substances. pH measurements help in quality control, scientific research, environmental monitoring, and many other applications.
- How often should I calibrate my pH meter?
- It is generally recommended to calibrate your pH meter before each use or at least once a day. However, the frequency of calibration may vary depending on the specific requirements of your application and the manufacturer's guidelines.
- Can I use distilled water for calibration?
- Distilled water is not suitable for pH calibration as it has a neutral pH. Calibration solutions with known pH values should be used to calibrate the pH meter and establish accurate measurements.
- How do I know if my pH meter needs calibration?
- If you notice inconsistent readings, significant deviations from expected values, or if it has been a while since the last calibration, it's a good indication that your pH meter needs calibration. Regular calibration ensures accurate and reliable pH measurements.
- Can I reuse calibration solutions?
- Calibration solutions should not be reused once they have been exposed to the pH meter or the environment. Reusing them can lead to contamination and inaccurate calibration. It is best to use fresh calibration solutions for each calibration.
- How long does a pH electrode last?
- The lifespan of a pH electrode depends on various factors such as usage frequency, sample composition, and maintenance. With proper care and maintenance, a pH electrode can last for several months to a couple of years. Regular calibration and cleaning help prolong the electrode's lifespan.
- Can I immerse the pH meter completely in the sample?
- It depends on the design and specifications of the pH meter. Some pH meters have waterproof or splash-proof features and can be fully immersed in the sample, while others may have limitations. Check the manufacturer's guidelines to determine the appropriate immersion depth for your pH meter.
- How can I clean the pH electrode?
- Rinse the electrode with distilled water after each use to remove any residue. For more thorough cleaning, you can use a specialized electrode cleaning solution or a mild detergent solution. Follow the manufacturer's instructions for cleaning and maintenance procedures.
- What is the effect of temperature on pH measurements?
- Temperature can significantly affect pH readings. pH meters with temperature compensation features adjust the pH value based on the sample's temperature. It is important to use temperature-compensated pH meters or apply manual temperature corrections to obtain accurate pH measurements.
- Can pH meters measure the pH of non-aqueous solutions?
- pH meters are primarily designed for measuring the pH of aqueous solutions. They may not provide accurate readings for non-aqueous solutions. In such cases, specialized pH meters or electrodes designed for non-aqueous solutions should be used.
References
https://www.joom.com/nl/products/5d5f8daf36b54d01011695ee
https://www.olibetta.nl/milwaukee/mw101-pro-ph-meter
https://www.indiamart.com/proddetail/digital-ph-meter-avi-make-6245810833.html
https://www.plantbiologic.com/products/digital-ph-meter-test-your-food-plot
https://soapandmore.ca/fr/products/ph-meter
https://etkeni.com/measuring/portable-ph-meter/
https://420shop.nl/en/ph-ec-combi-meter-3465.html
https://www.labcompare.com/Chemical-Analysis-Equipment/960-Benchtop-pH-Meter/
Recent Posts
-
Booster Pump Troubleshooting and Maintenance: How to Fix and Prevent Common Issues
1. Introduction Imagine turning on your faucet only to be greeted with a weak trickle of water when …22nd Apr 2025 -
Energy-Efficient Booster Pumps: Selection and Tips for Maximizing Performance
1. Introduction Imagine never having to deal with fluctuating water pressure, noisy pumps, or skyroc …19th Apr 2025 -
Booster Pumps for Sustainable Water Systems: Irrigation and Rainwater Harvesting Solutions
1. Introduction Water scarcity is no longer a distant threat—it’s a reality affecting millions …16th Apr 2025




